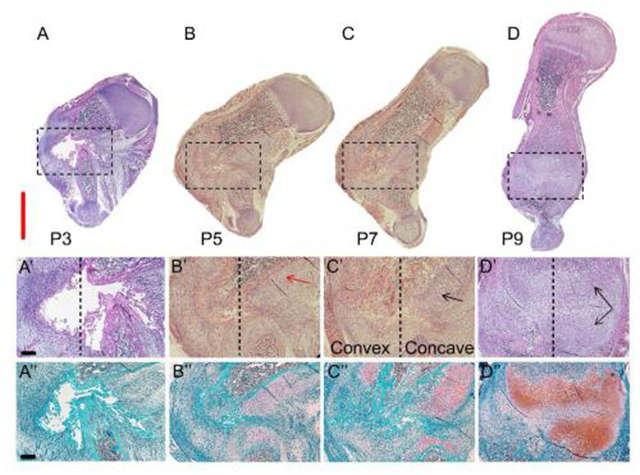
(A-D) This image shows H&E and Safranin O/Fast Green staining of sections through the fracture site during the healing process (P3–P9). Scale bar, 2 mm. (A'-D') Magnifications of the boxed areas are in the upper panel. The dashed lines separate between the concave side (on the right) and convex side. The red arrow indicates cells appearing as chondrocytes, and black arrows indicate cells appearing as hypertrophic chondrocytes. (A''-D'') As indicted by Safranin O staining (pink-to-red colors), as the healing process progresses the soft callus is increasingly composed of cartilage. Scale bar, 200 mm. Credit: Developmental Cell, Rot et al.
If you've ever broken a bone, there's a good chance you needed surgery, braces, or splints to realign the bone. Severe fractures in infants, on the other hand, can heal on their own through a process that has eluded scientists. A study published by Cell Press on October 27 in Developmental Cell reveals that a fractured arm bone in newborn mice can rapidly realign through a previously unknown mechanism involving bone growth and muscle contraction. The findings provide new insights into how human infants and other young vertebrates may repair broken bones and pave the way for more effective treatment strategies.
"Evolution has created a robust mechanism of bone regeneration, which may explain how wild animals can survive traumatic bone injuries," says senior study author Elazar Zelzer of the Weizmann Institute of Science. "Further investigation of the newly found regeneration program could lead to alternative approaches for the treatment of fractures that do not respond well to current practices."
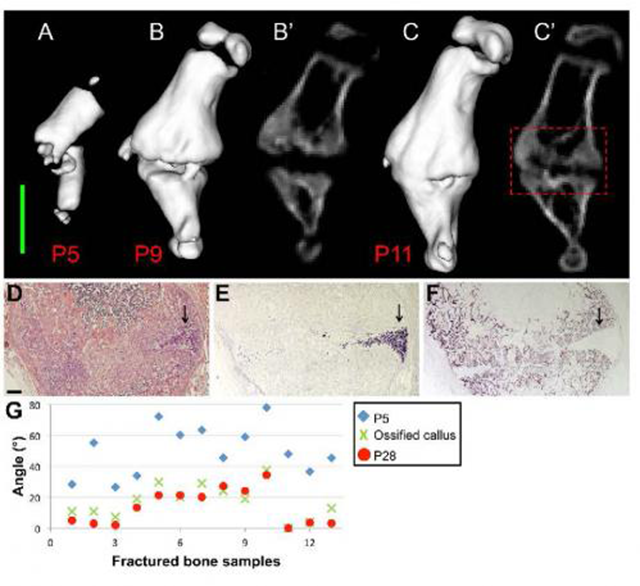
These are 3-D in vivo CT images of a fractured humerus at P5 (A), P9 (B), and P11 (C). (B' and C') 2-D in vivo CT images of the same bone at P9 and P11, respectively. Scale bar, 2 mm. Magnifications of the concave side of the fracture, demarcated by a red dashed box in (C'). The presence of chondrocytes (indicated by black arrows) is demonstrated by H&E staining (D) and by in situ hybridization for Col10a1 (E). The progression of the mineralization process from the convex toward the concave side is shown by the osteoblastic marker Bsp (F). Scale bar, 200 mm. (G) Scatter plot showing the angle between the two fragments of all fractured humeri (n = 13), as measured at P5 (blue diamond), during callus ossification (P11-P15, green X) and at P28 (red circle). Credit: Developmental Cell, Rot et al.
The bone is one of the few organs that can regenerate itself in vertebrates. Although spontaneous regeneration occurs in infants, adults require interventions to return the bone to a straight position, as well as stabilization with metallic hardware or a cast. But standard treatment protocols have several side effects, including muscle atrophy and joint stiffness. Zelzer and his team suspected that a better understanding of natural regeneration in infants could help to improve interventions for fractured bones in adults.
In the new study, the researchers found that a fractured arm bone in newborn mice rapidly realigned through substantial movement of bone fragments rather than through bone remodeling – a slower process involving the simultaneous formation of new bone on one side and erosion of existing bone on the opposite side. "This finding challenges the traditional view of fracture healing and introduces an entirely new stage of bone repair to the classical four-stage model," Zelzer says.
The realignment process was driven by bone growth, which acted like a mechanical jack to generate the opposing forces required to straighten the two bone fragments. Moreover, treatment with a drug that paralyzed the muscles surrounding the fracture prevented normal bone growth and bone realignment, suggesting that muscle contraction plays a critical role in the repair process.
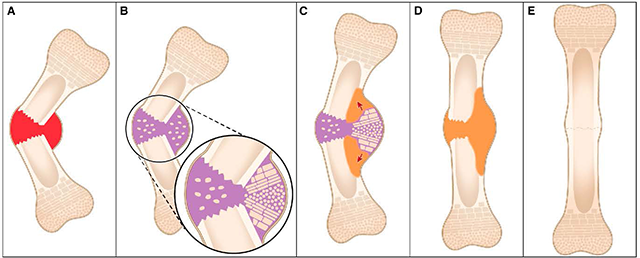
(A) Healing begins with hematoma and inflammation at the fracture site. (B) Soft callus (purple) is formed, which we show to organize as a bidirectional growth plate at the concave side of the fracture site. (C) The two growth plates drive growth in opposite directions. The result is a jack-like mechanical effect that moves the fragments toward straightening (red arrows). (D) Ossification produces hard callus (orange). (E) The shape of the bone is fine-tuned by remodeling. Credit: Developmental Cell, Rot et al.
"Integrating this new knowledge into the current approach may improve future treatment," Zelzer says. "For example, treatment protocols may include age-based protocols and shorter periods of rigid immobilization to allow participation of muscle force in the healing process."
