The typical Hollywood action hero skirts death for a living. Time and again, scores of bad guys shoot at him from multiple directions but miss by a hair. Cars explode just a fraction of a second too late for the fireball to catch him before he finds cover. And friends come to the rescue just before a villain's knife slits his throat. If any one of those things happened just a little differently, the hero would be hasta la vista, baby. Yet even if we have not seen the movie before, something tells us that he will make it to the end in one piece.
In some respects, the story of our universe resembles a Hollywood action movie. Several physicists have argued that a slight change to one of the laws of physics would cause some disaster that would disrupt the normal evolution of the universe and make our existence impossible. For example, if the strong nuclear force that binds together atomic nuclei had been slightly stronger or weaker, stars would have forged very little of the carbon and other elements that seem necessary to form planets, let alone life. If the proton were just 0.2 percent heavier than it is, all primordial hydrogen would have decayed almost immediately into neutrons, and no atoms would have formed. The list goes on.
The laws of physics—and in particular the constants of nature that enter into those laws, such as the strengths of the fundamental forces—might therefore seem finely tuned to make our existence possible. Short of invoking a supernatural explanation, which would be by definition outside the scope of science, a number of physicists and cosmologists began in the 1970s to try solving the puzzle by hypothesizing that our universe is just one of many existing universes, each with its own laws. According to this "anthropic" reasoning, we might just occupy the rare universe where the right conditions happen to have come together to make life possible.

Amazingly, the prevailing theory in modern cosmology, which emerged in the 1980s, suggests that such "parallel universes" may really exist—in fact, that a multitude of universes would incessantly pop out of a primordial vacuum the way ours did in the big bang. Our universe would be but one of many pocket universes within a wider expanse called the multiverse. In the overwhelming majority of those universes, the laws of physics might not allow the formation of matter as we know it or of galaxies, stars, planets and life. But given the sheer number of possibilities, nature would have had a good chance to get the "right" set of laws at least once.
Our recent studies, however, suggest that some of these other universes—assuming they exist—may not be so inhospitable after all. Remarkably, we have found examples of alternative values of the fundamental constants, and thus of alternative sets of physical laws, that might still lead to very interesting worlds and perhaps to life. The basic idea is to change one aspect of the laws of nature and then make compensatory changes to other aspects.
Our work did not address the most serious fine-tuning problem in theoretical physics: the smallness of the "cosmological constant," thanks to which our universe neither recollapsed into nothingness a fraction of a second after the big bang, nor was ripped part by an exponentially accelerating expansion. Nevertheless, the examples of alternative, potentially habitable universes raise interesting questions and motivate further research into how unique our own universe might be.
The Weakless Way of Life
The conventional way scientists find out if one particular constant of nature is finely tuned or not is to turn that "constant" into an adjustable parameter and tweak it while leaving all other constants unaltered. Based on their newly modified laws of physics, the scientists then "play the movie" of the universe—they do calculations, what-if scenarios or computer simulations—to see what disaster occurs first. But there is no reason why one should tweak only one parameter at a time. That situation resembles trying to drive a car by varying only your latitude or only your longitude, but not both: unless you are traveling on a grid, you are destined to run off the road. Instead one can tweak multiple parameters at once.
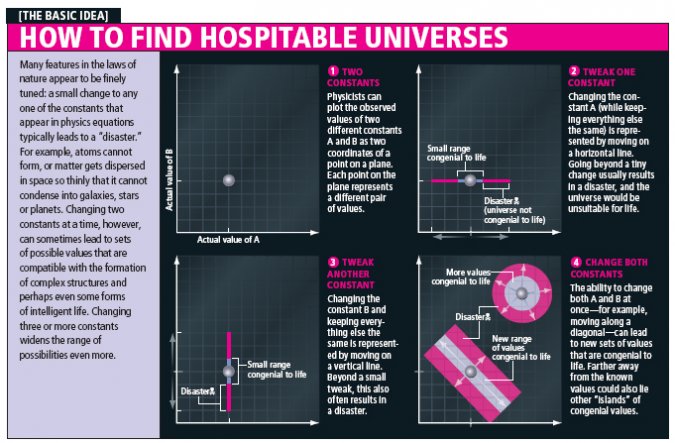
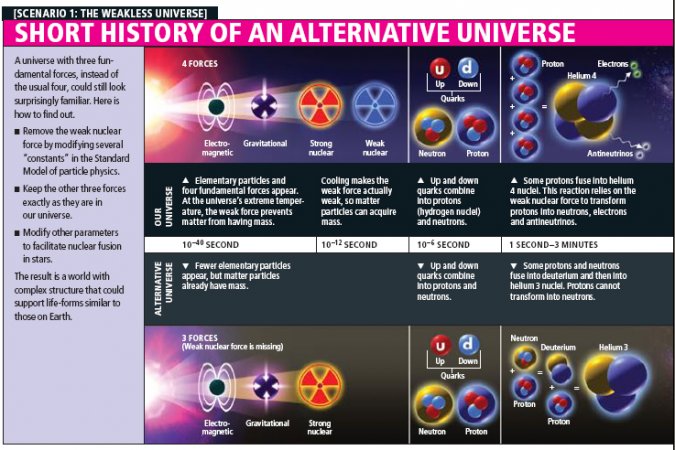
To search for alternative sets of laws that still give rise to complex structures capable of sustaining life, one of us (Perez) and his collaborators did not make just small tweaks to the known laws of physics: they completely eliminated one of the four known fundamental forces of nature.
By their very name, the fundamental forces sound like indispensable features of any self-respecting universe. Without the strong nuclear force to bind quarks into protons and neutrons and those into atomic nuclei, matter as we know it would not exist. Without the electromagnetic force, there would be no light; there would also be no atoms and no chemical bonds. Without gravity, there would be no force to coalesce matter into galaxies, stars and planets.
The fourth force, the weak nuclear force, has a subtler presence in our everyday life but still has played a major role in the history of our universe. Among other things, the weak force enables the reactions that turn neutrons into protons, and vice versa. In the first instants of the big bang, after quarks (among the first forms of matter to appear) had united in groups of three to form protons and neutrons, collectively called baryons, groups of four protons were then able to fuse together and become helium 4 nuclei, made of two protons and two neutrons. This so-called big bang nucleosynthesis took place a few seconds into the life of our universe, when it was already cold enough for baryons to form but still hot enough for the baryons to undergo nuclear fusion. Big bang nucleosynthesis produced the hydrogen and helium that would later form stars, where nuclear fusion and other processes would forge virtually all other naturally occurring elements. And to this day, the fusion of four protons to make helium 4 continues inside our sun, where it produces most of the energy that we receive from it.
Without the weak nuclear force, then, it seems unlikely that a universe could contain anything resembling complex chemistry, let alone life. Yet in 2006 Perez's team discovered a set of physical laws that relied on only the other three forces of nature and still led to a congenial universe.
Eliminating the weak nuclear force required several modifications to the so-called Standard Model of particle physics, the theory that describes all forces except gravity. The team showed that the tweaks could be done in such a way that the behavior of the other three forces—and other crucial parameters such as the masses of the quarks—would be the same as in our world. We should stress that this choice was a conservative one, intended to facilitate the calculation of how the universe would unfold. It is quite possible that a wide range of other "weakless" universes exist that are habitable but look nothing like our own.
In the weakless universe, the usual fusing of protons to form helium would be impossible, because it requires that two of the protons convert into neutrons. But other pathways could exist for the creation of the elements. For example, our universe contains overwhelmingly more matter than antimatter, but a small adjustment to the parameter that controls this asymmetry is enough to ensure that the big bang nucleosyn thesis would leave behind a substantial amount of deuterium nuclei. Deuterium, also known as hydrogen 2, is the isotope of hydrogen whose nucleus contains a neutron in addition to the usual proton. Stars could then shine by fusing a proton and a deuterium nucleus to make a helium 3 (two protons and one neutron) nucleus.
Such weakless stars would be colder and smaller than the stars in our own universe. According to computer simulations by astrophysicist Adam Burrows of Princeton University, they could burn for about seven billion years—about the current age of our sun—and radiate energy at a rate that would be a few percent of that of the sun.
Next Generation
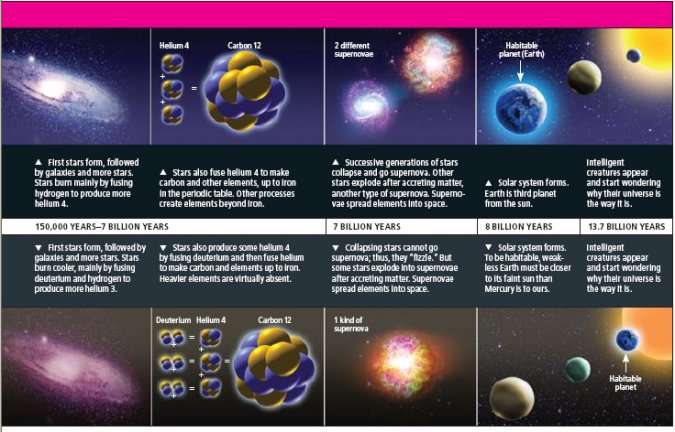
Just like stars in our universe, weakless stars could synthesize elements as heavy as iron through further nuclear fusion. But the typical reactions that in our stars lead to elements beyond iron would be compromised, primarily because few neutrons would be available for nuclei to capture to become heavier isotopes, the first step in the formation of heavier elements. Small amounts of heavy elements, up to strontium, might still be synthesized inside weakless stars by other mechanisms.
In our universe, supernova explosions disperse the newly synthesized elements into space, and synthesize more of the elements themselves. Supernovae can be of several types: in the weakless universe, the supernova explosions caused by collapsing ultramassive stars would fail, because it is the emission of neutrinos, produced via the weak force interactions, that transmits energy out of a star's core so as to sustain the shock wave that is causing the explosion. But a different type of supernova—the thermonuclear explosion of a star triggered by accretion, rather than by gravitational collapse—would still take place. Thus, elements could be dispersed into interstellar space, where they could seed new stars and planets.
Given the relative coldness of the weakless stars, a weakless Earth-like body would have to be about six times closer to its sun to stay as warm as our own Earth. To the inhabitants of such a planet, the sun would look much bigger. Weakless Earths would be significantly different from our own Earth in other ways. In our world, plate tectonics and volcanic activity are powered by the radioactive decay of uranium and thorium deep within Earth. Without these heavy elements, a typical weakless Earth might have a comparatively boring and featureless geology—except if gravitational processes provided an alternative source of heating, as happens on some moons of Saturn and Jupiter.
Chemistry, on the other hand, would be very similar to that of our world. One difference would be that the periodic table would stop at iron, except for extremely small traces of other elements. But this limitation should not prevent life-forms similar to the ones we know from evolving. Thus, even a universe with just three fundamental forces could be congenial to life.
Another approach, pursued by the other of us (Jenkins) and his collaborators, searches for alternative sets of laws by making smaller tweaks to the Standard Model than in the case of the weakless universe, though still involving multiple parameters at once. In 2008 the group studied to what extent the masses of the three lightest of the six quarks—called the up, down and strange quarks—may vary without making organic chemistry impossible. Changing the quark masses will inevitably affect which baryons and which atomic nuclei can exist without decaying quickly. In turn, the different assortment of atomic nuclei will affect chemistry.
Quarky Chemistry
It seems plausible that intelligent life (if it is not very different from us) requires some form of organic chemistry, which is by definition the chemistry that involves carbon. The chemical properties of carbon follow from the fact that its nucleus has an electric charge of 6, so that six electrons orbit in a neutral carbon atom. These properties allow carbon to form an immense variety of complex molecules. (The suggestion often made by science-fiction writers that life could instead be based on silicon—the next element in carbon's group in the periodic table—is questionable: no silicon-based molecules of any significant degree of complexity are known to exist.) Furthermore, for complex organic molecules to form, elements with the chemistry of hydrogen (charge 1) and oxygen (charge 8) need to be present. To see if they could maintain organic chemistry, then, the team had to calculate whether nuclei of charge 1, 6 or 8 would decay radioactively before they could participate in chemical reactions [see box on next page].
The stability of a nucleus partly depends on its mass, which in turn depends on the masses of the baryons it is made of. Computing the masses of baryons and nuclei starting from the masses of the quarks is extremely challenging even in our universe. But after tweaking the intensity of the interaction between quarks, one can use the baryon masses measured in our universe to estimate how small changes to the masses of the quarks would affect the masses of nuclei.
In our world, the neutron is roughly 0.1 percent heavier than the proton. If the masses of the quarks were changed so that the neutron became 2 percent heavier than the proton, no long-lived form of carbon or oxygen would exist. If quark masses were adjusted to make the proton heavier than the neutron, then the proton in a hydrogen nucleus would capture the surrounding electron and turn into a neutron, so that hydrogen atoms could not exist for very long. But deuterium or tritium (hydrogen 3) might still be stable, and so would some forms of oxygen and carbon. Indeed, we found that only if the proton became heavier than the neutron by more than about 1 percent would there cease to be some stable form of hydrogen.
With deuterium (or tritium) substituting for hydrogen 1, oceans would be made of heavy water, which has subtly different physical and chemical properties from ordinary water. Still, there does not appear to be a fundamental obstacle in these worlds to some form of organic life evolving.
In our world, the third-lightest quark—the strange quark—is too heavy to participate in nuclear physics. But if its mass were reduced by a factor of more than about 10, nuclei could be made not just of protons and neutrons but also of other baryons containing strange quarks.
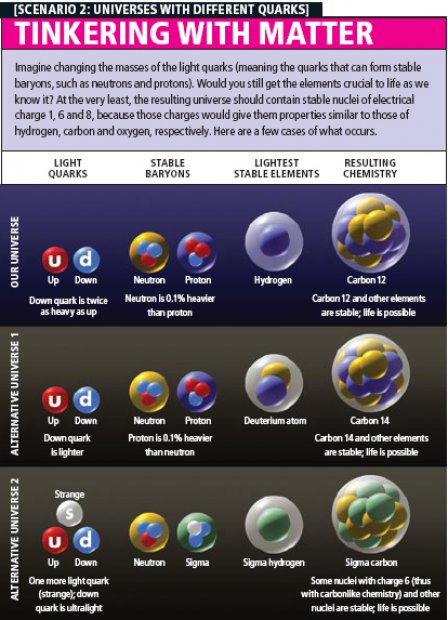
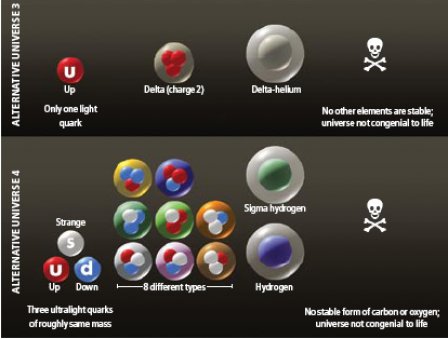
For example, the team identified a universe in which the up and strange quark would have roughly the same mass, whereas the down quark would be much lighter. Then atomic nuclei would not be made of protons and neutrons but instead of neutrons and another baryon, called the Σ– ("sigma minus"). Remarkably, even such a radically different universe would have stable forms of hydrogen, carbon and oxygen and therefore could have organic chemistry. Whether those elements would be produced abundantly enough for life to evolve somewhere within them is an unanswered question.
But if life can arise, it would again happen much like it does in our world. Physicists in such a universe might be puzzled by the fact that the up and strange quarks would have almost identical masses. They might even imagine that this amazing coincidence has an anthropic explanation, based on the need for organic chemistry. We know, however, that such an explanation would be wrong, because our world has organic chemistry even though the masses of the down and strange quarks are quite different.
On the other hand, universes in which the three light quarks had roughly the same masses would probably have no organic chemistry: any nucleus with more than a couple of units of electrical charge would decay away almost immediately. Unfortunately, it is very difficult to map out in detail the histories of universes whose physical parameters are different from our own. This issue requires further research.
String Landscaping
Fine-tuning has been invoked by some theoretical physicists as indirect evidence for the multiverse. Do our findings therefore call the concept of a multiverse into question? We do not think that this is necessarily the case, for two reasons. The first comes from observation, combined with theory. Astronomical data strongly support the hypothesis that our universe started out as a tiny patch of spacetime, perhaps as small as a billionth the size of a proton, which then went through a phase of rapid, exponential growth, called inflation. Cosmology still lacks a definitive theoretical model for inflation, but theory suggests that different patches could inflate at different rates and that each patch could form a "pocket" that can become a universe in its own right, characterized by its own values for the constants of nature [see "The Self-Reproducing Inflationary Universe," by Andrei Linde; Scientific American, November 1994]. Space between pocket universes would keep expanding so fast that it would be impossible to travel or send messages from one pocket to the next, even at the speed of light.
The second reason to suspect the existence of the multiverse is that one quantity still seems to be finely tuned to an extraordinary degree: the cosmological constant, which represents the amount of energy embodied in empty space. Quantum physics predicts that even otherwise empty space must contain energy. Einstein's general theory of relativity requires that all forms of energy exert gravity. If this energy is positive, it causes spacetime to expand at an exponentially accelerating rate. If it is negative, the universe would recollapse in a "big crunch." Quantum theory seems to imply that the cosmological constant should be so large—in the posi-tive or negative direction—that space would expand too quickly for structures such as galaxies to have a chance to form or else that the universe would exist for a fraction of a second before recollapsing.
One way to explain why our universe avoided such disasters could be that some other term in the equations canceled out the effects of the cosmological constant. The trouble is that this term would have to be fine-tuned with exquisite precision. A deviation in even the 100th decimal place would lead to a universe without any significant structure.
In 1987 Steven Weinberg, the Nobel Prize–winning theorist at the University of Texas at Austin, proposed an anthropic explanation. He calculated an upper bound on the value of the cosmological constant that would still be compatible with life. Were the value any bigger, space would expand so quickly that the universe would lack the structures that life requires. In a way, then, our very existence predicts the low value of the constant.
Then, in the late 1990s, astronomers discovered that the universe is indeed expanding at an accelerating rate, pushed by a mysterious form of "dark energy." The observed rate implied that the cosmological constant is positive and tiny—within the bounds of Weinberg's prediction—meaning that dark energy is very dilute.
Thus, the cosmological constant seems to be fine-tuned to an exceptional degree. Moreover, the methods our teams have applied to the weak nuclear force and to the masses of quarks seem to fail in this case, because it seems impossible to find congenial universes in which the cosmological constant is substantially larger than the value we observe. Within a multiverse, the vast majority of universes could have cosmological constants incompatible with the formation of any structure.
A real-world analogy—as opposed to an action-movie one—would be to send thousands of people trekking across a mountainous desert. The few who make it out alive might tell stories full of cliffhangers, encounters with poisonous snakes, and other brushes with death that would seem too close to be realistic.
Theoretical arguments rooted in string theory—a speculative extension of the Standard Model that attempts to describe all forces as the vibrations of microscopic strings—seem to confirm such a scenario. These arguments suggest that during inflation the cosmological constant and other parameters could have taken a virtually limitless range of different values, called the string theory landscape [see "The String Theory Landscape," by Raphael Bousso and Joseph Polchinski; Scientific American, September 2004].
Our own work, however, does cast some doubt on the usefulness of anthropic reasoning, at least beyond the case of the cosmological constant. It also raises important questions. For example, if life really is possible in a weakless universe, then why does our own universe have a weak force at all? In fact, particle physicists consider the weak force in our universe to be, in a sense, not weak enough. Its observed value seems unnaturally strong within the Standard Model. (The leading explanation for this mystery requires the existence of new particles and forces that physicists hope to discover at the newly opened Large Hadron Collider at CERN near Geneva.)
As a consequence, many theorists expect that most universes would have weak interactions that are so feeble as to be effectively absent. The real challenge, then, may be to explain why we do not live in a weakless universe.
Eventually only a deeper knowledge of how universes are born can answer such questions. In particular, we may discover physical principles of a more fundamental level that imply that nature prefers certain sets of laws over others.
We may never find any direct evidence of the existence of other universes, and we certainly will never get to visit one. But we may need to learn more about them if we want to understand what is our true place in the multiverse—or whatever it is that is out there.
