Between April 2004 and February 2005, 28 men with prostate cancer showed up at three Canadian hospitals for one-time injections of an experimental drug designed to eradicate their deadly tumors. Radiation had already failed them.
By December tumors in the earliest-treated patients had shrunk by as much as 84%. But the real test began in March, when doctors started studying tissue samples. If they find the tumors are gone or reduced to a manageable level, it may indicate that the drug is reliable over time, and researchers could be on their way to a radical advancement in localized prostate cancer treatment.
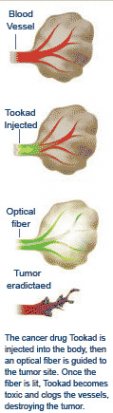
The drug, trademarked Tookad (a Hebrew word suggesting "the warmth of light"), is an innovative twist within the established cancer-drug class called photodynamic therapies. These drugs work their way through the bloodstream but become toxic only when exposed to light. When doctors shine a laser light onto either the skin or an internal tumor using catheter-inserted optical fibers, the drugs kill just the illuminated tissue and leave unexposed tissue relatively undamaged.
Tookad was designed to eradicate bulkier tumors, with fewer side effects than those caused by existing photodynamic drugs. Once illuminated by laser light, it clogs the tiny blood vessels feeding the tumors, starving the cancer of oxygen and nutrients. If it proves successful, Tookad will be a welcome addition to existing prostate treatments such as surgery, freezing, and radiation, which can cause impotence and incontinence or kill healthy cells. So far Tookad has produced none of these side effects.
Photosensitizers have been used for a decade against esophageal, bladder, lung, and skin cancers, but they leave the body so slowly that patients must avoid outdoor light for up to six weeks after treatment or risk skin burns. Tookad degrades more easily in the liver and exits the body within hours.
"This is one of the most promising treatments for recurrent prostate cancer after radiation I've ever seen," says John Trachtenberg, director of the Prostate Centre at Princess Margaret Hospital in Toronto and principal investigator for Tookad's worldwide trials, which are also under way in Britain, France, and Israel. Results are due early next year. Successful results will also spotlight Steva NV, the family-owned Dutch company that has licensed Tookad and poured tens of millions of dollars into its development since 1996.
Tookad was developed over several years by biologist Yoram Salomon and plant photochemist Avigdor Scherz of the Weizmann Institute of Science in Israel. Their breakthrough was in using a bacteria-derived photosensitive agent instead of the traditionally used heme, the red pigment found in hemoglobin. Heme can be activated by visible light at a wavelength of 630 nanometers, which can penetrate thin tumors but nothing much thicker. Tookad is derived from bacteriochlorophyll, the bacterial equivalent of the green plant pigment chlorophyll, which drives photosynthesis. It is activated by near-infrared light at a wavelength of 763 nanometers, long enough to penetrate deeper, up to 2 centimeters, into the tissue. Lighting up several fibers, doctors can treat even larger tumors.
"People tried chlorophyll before but failed since they used the native green pigment from plants," says Salomon, a professor with the Institute's biological regulation department. "Avigdor and I modified bacteriochlorophyll to adapt it as a drug."
Tookad also fits into a burgeoning class of vessel-targeting cancer drugs that includes Genentech's successful Avastin. But, while Avastin requires patients to remain on the drug, Tookad can be administered once. In animal tests it has shown the ability to work against tumors that have become chemotherapy resistant; early work is under way on liver and lung tumors. Says Solomon Hamburg, a clinical professor of medicine at UCLA Medical Center: "This is not the kind of technology that will revolutionize outcomes. But it may change options. If I was 65 and had prostate cancer, and I had a choice between this drug and surgery or radiation, I'd strongly consider using this drug first." "The goal is the same: to block blood flow to the tumor," says Salomon. "But we blow up the bridges. They [Avastin developers] slowly narrow the streets until no one can go through. If you stop their drug, the vessels grow back."
