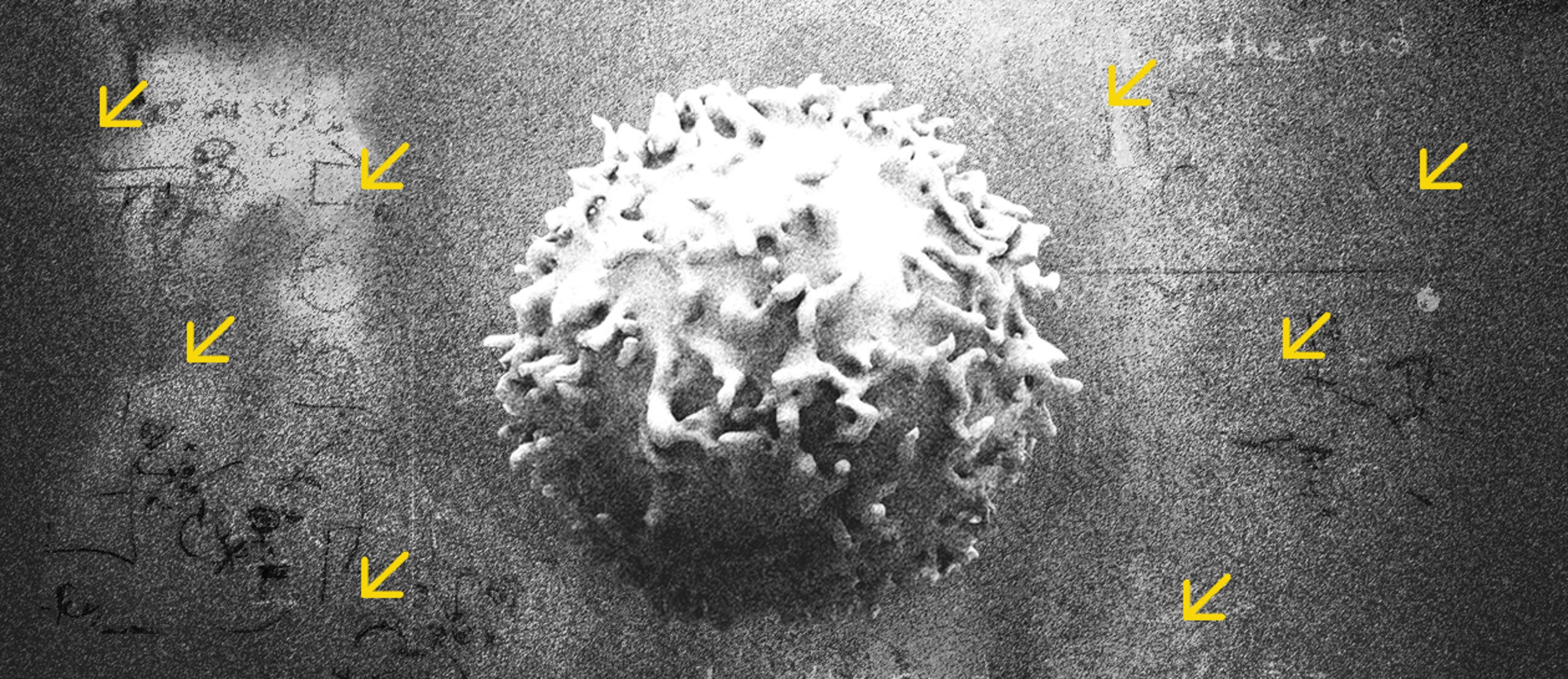
These are coronavirus days, and very few people can be seen in the narrow corridors of the Weizmann Institute of Science in Rehovot. We’re in the molecular immunology laboratory, located in one of the institute’s old buildings. It’s a simple place, the instruments are banal and dull. It looks like any lab in the world, one of thousands. But a miracle is occurring between the walls here. In the small details. The smallest.
The lab is mirrored by the person who heads it. Ido Amit, 49, speaks in a gentle voice. He’s stingy with words when asked about matters that don’t pertain to science. Occasionally he stands up in order to draw something on the whiteboard that will help explain what he’s saying, and when he gets enthusiastic about a particularly exciting discovery or development his speech speeds up a little. Prof. Amit’s tone of voice hardly betrays the breakthrough nature of what he’s saying. It’s hard to imagine that he is promising a revolution that will be able to transform the world of medicine.
The history of medicine is rife with cases of publicity-hunting scientists and entrepreneurs who have promised a cure for cancer. Most were soon forgotten, a few still have starring roles in cautionary tales about the limits of human knowledge. With a clear awareness of this, the immunologist chooses his words carefully. Nevertheless, he replies repeatedly and unhesitatingly that he is convinced that his discoveries and the technologies he has developed will foment a revolution.
Amit’s revelations have emerged from a singular research approach, which has characterized his career from the get-go. The idea sounds simple: first, to break down the subject of the research (in this case, cancer) into its basic components (the cells), then to try to understand how they work together – and afterward to decipher what has changed when something goes wrong. “Like with fixing a radio,” he says, summing up simply his research philosophy.
That approach led him to found, together with his colleagues, a field of research for the study of individual cells. The research methods they employ, known as “single-cell genomics,” are considered a quantum leap in the field and in recent years have come into wide use in laboratories worldwide. Many scientists are currently involved in the development and application of these methods, and broad agreement exists regarding their ability to provide an impetus for both scientific and practical research.
Indeed, Amit’s work is unique not only because of the technology he has created but because of the way he applies it. He is not engaged solely in developing technologies and then moving on. He uses the technologies to better understand diseases. Above all, cancer.
The professor’s work draws on the great revolution in the study of cancer at the beginning of this century – for which the Nobel Prize in Medicine was awarded two years ago – based on the discovery that the immune system can be harnessed to fight malignant tumors. There is one type of cancer for which this finding was particularly dramatic: melanoma. Today, more than half of those who contract skin cancer recover completely, thanks to immunotherapy treatment. However, what works for about 60 percent of those who are ill with one type of cancer, is not effective for the overwhelming majority of those who suffer from other types of the disease.
With the aid of the advanced tools developed in their lab that enable scientists to study individual cells, Amit and his colleagues discovered how cancer succeeds in disrupting the activity of the immune system. The researchers’ methods reveal how the majority of malignant tumors continue to evade the immune system, even when it is induced with our most sophisticated tools to attack them.
Amit and his team are currently taking part in a lengthy series of studies dealing with different types of cancer (such as those attacking the lungs, skin, breast and bone marrow) with leading pharmaceutical companies and with some of the world’s senior oncologists. In all these projects, a close translational connection is maintained from bedside to bench and to bedside again, and the insights gained breach the boundaries of basic science. They have a single goal: to parlay the discoveries made into the next generation of immunotherapy, and thus enable the success with melanoma to be replicated with the other types of cancer.
Amit believes that the first treatments based on his methods will be approved for clinical use for cancer patients in another two to four years. He thinks that within the coming decade the breakthrough methods he and his colleagues are working on will make the move from research laboratories to the clinical laboratories. This will make possible a deeper understanding of every malignant tumor in every patient, and prescription of the most suitable and effective combination of medications to cure the disease.
But as ambitious as this sounds, the Weizmann Institute scientist has plans that go beyond finding effective treatments for cancer. On the basis of his findings, and with the aid of the singular technology he is using, medications have been developed to inhibit and contain the development of Alzheimer’s and autoimmune diseases. The effectiveness of the substances in question is currently under examination in clinical experiments; the findings, he says, are promising.
And there is more: Amit’s vision is even more expansive than his hope to find medications for treating cancer and Alzheimer’s. He believes that his methods will be able to effect a dramatic improvement in the development of medicine in general. With the use of single-cell genomics, the process of developing a new drug, whose cost can exceed more than $1 billion for a single new medication, can be far more precise, focused and smart.

‘The world opened up’
As in a classic Zionist story, Amit’s view to science was shaped amid the fields of the Jezreel Valley, where he grew up. He was born on Kibbutz Hatzor, near Ashdod, but his parents left the kibbutz when he was 1, because they didn’t want their son growing up in a communal children’s house. Moving north, they settled on Kibbutz Yizre’el, which had already done away with that element of kibbutz life.
“I wanted to understand how field crops grow,” he relates, explaining the connection between the natural surroundings he saw and his father’s profession as an electronics and computer engineer. “I tried to think about what my father did and translate it into the biological world. That line of thought – how we can create a link between mechanical engineering and design, and the biological world – always intrigued me.”
Following his military service in an elite unit in the Golani infantry brigade, Amit earned his bachelor’s and master’s degrees in biology at Bar-Ilan University, then went on to doctoral studies at the Weizmann Institute in the laboratory of Prof. Yossi Yarden. “The world opened up for me there,” he recalls. The lab specialized in the study of a receptor that appears on cells in a variety of body tissues, its role being to encourage cell growth and division. Cancer exploits this process: By greatly multiplying the quantity of receptors, it causes uncontrollable cell growth.
“The approach in the lab was classically mechanistic, hypothesis-based,” Amit says, referring to one of the major problems he detects in research in his field. “To get funding for research you first of all have to present a hypothesis – ‘I think that X does Y, and I want to measure it.’ That approach is very characteristic of biological research today. But if you think about it, that isn’t what Darwin, the father of the idea of evolution, did. He boarded his ship, the Beagle, and sailed into open seas. His observations were what led him to think of evolution.”
The problem with today’s approach, the professor continues, is that, in general, you find what you’re looking for. “But the fascinating thing about science is to be surprised. We are constantly limited by the thoughts in our head, and the thoughts in our head depend on what we read beforehand. So progress is generally incremental and very predictable. There have been wonderful ideas and breakthroughs that were achieved like that, but the progress is gradual. And maybe we’re only looking at the tip of the iceberg, and we tend to confirm what we are looking for. I wanted to set sail with the Beagle into the open seas.”
Amit notes that when he worked in the lab, he developed, together with Profs. Yarden and Ami Citri, “a simple idea, but a bit different, one that makes it possible to set sail.” When a cell receptor receives a signal, two parallel processes occur, and it’s the first that causes the cell to divide. However, at the same time, the activation of the receptor should signal the cell to block the original signal, because otherwise the process will continue uncontrollably.
Amit: “There was evidence of a process like that, but I wanted to study it systematically. I wanted to measure all the RNA [molecules of the genetic material that serve the cells for various purposes] the cell manufactures before and after receiving the transmission.”
‘Lit up’ genes
Today it is possible to measure all the RNA in an individual cell, in part thanks to methods developed by Amit and his colleagues. However, when he was working on his doctoral thesis, in 2000, those tools were not yet available. Pioneering research at that time enabled DNA sequences to be printed on small glass slides, to take RNA samples collected from cells before and after their exposure to a stimulus, to label them with florescent probes, and then to hybridize them with the slides.
By that means, Amit and other scientists in Yarden’s laboratory could discern which RNA molecules were active before the cell was exposed to a stimulus, which of them were active after an hour, after two hours and so on – by examining which genes “lit up” on the slide.
“If we think of a radio, we understood that the button to turn it on was apparently here, the antenna was perhaps there and this looked like the speaker. Nothing was proven, but you can start to generate hypotheses on the basis of the data, trying to understand causality,” Amit relates. “We found that activation of the receptor sets in motion a chain of processes. A real transmission tower that relays to another transmission tower that relays to a third, which in the end creates the cellular decisions. And we have a way to decipher those transmissions, like collecting intelligence about the cell’s transmissions.
“I grew more confident that the approach of ‘venturing into the open seas’ had potential,” he adds. “And the second thing I learned there, which was crucial for me, was the tremendous importance of technology. However brilliant we may be – and there is no end of brilliant people in biology all over the world – what really enables you to move science ahead is new technologies. Because they enable you to see something that hadn’t been seen before.”
In 2007, after obtaining his Ph.D., Amit, together with his wife and their children, moved to Boston, where he hooked up with Prof. Nir Hacohen, an Israeli-born immunologist and geneticist from Harvard and from a Boston hospital. Together they proposed the following idea: to examine a group of immune cells and to “turn off” one gene at a time. In each such experiment, they would examine what changes in the immune cells’ reaction to different pathogens – against which they were supposed to act – and thereby understand the role of each gene in the intricate gene circuit.
“I would take a group of 20,000 cells, erase the gene in all of them, and ask what was happening in them in comparison to those I didn’t erase,” Amit explains. “I would try to cut off each wire after each wire in the radio and check what changed in its functioning.”
He hadn’t yet developed the technology that would enable him to explore individual cells, but even 20,000 cells constituted a very small amount of material. Accordingly, he and his associates needed to develop new analytical and technological methods.
They drew on facial-recognition algorithms. Just as such an algorithm can identify us on the basis of a small number of characteristics, the algorithms he used focused on 200 genes in the human DNA, in which a change in their “signature” would make it possible to detect different responses among the immune cells. The beta version didn’t succeed, but after overcoming a few obstacles they had the technology to examine how different cells in the immune system react to each other.
‘Yin and yang’
“There is a constant yin and yang in the immune system,” Amit notes. “The body has to make decisions all the time.” Is there foreign matter in it? And if so, how to cope with it? If the immune system detects a bacterium between the cells, additional cells need to be mobilized in order to fight it. But if it’s a virus that has already invaded the body’s cells, the immune system needs to eliminate the affected cells before the virus has been able to replicate itself. “And balance has to be maintained at all times,” he notes. If the immune system reacts too intensely, an autoimmune response will develop; if the reaction is weak, the invader will spread within the body.
Using the new technology they had developed, the scientists discovered which information processing the immune system cells carry out. The researchers showed how these computations enable the immune system to respond to a particular stimulus identically, time after time. These discoveries bore practical significance: understanding the operations performed by the cells might be of help in developing more effective vaccines or in calming the system when it is operating too intensively.
“The post-doc approached its end. It was very successful, a great many articles were published and it was a wonderful family experience as well. But then I started to think,” Amit recalls. “We had done all these studies in vitro, external to the organism. Our assumption was that we were investigating identical cells. But when I started trying to translate the insights into living model organisms, I saw that when one looked inside the mice, the richness of the cells was far greater.”
The first problem was the tools that were used to differentiate between the different immune cells. To understand them, we need to go back a little in time – 200 years, to be precise. That was the first time the development of the microscope made it possible to view immune cells directly. It turned out that some cells were identifiable by their shapes; some are long and narrow, others have multiple “arms.” There are cells that can be differentiated by their activity (like immune cells whose role is to “eat” waste). But shape and activity can change even within the life of a single cell. And there are many cell types that look the same under the microscope but their function is completely different.
The major revolution in this realm occurred in the 1980s and the 1990s, when scientists discovered in parallel how to generate antibodies that are linked to a particular type of immune cell, and the technology to accurately detect and sort these cells. It then became possible to manufacture antibodies that would enable the isolation of many types of immune cells, including T cells – the immune cells that detect and fight against wayward cells, including those that become cancerous.
In general, the technology involved adding the antibodies, which had been colored with fluorescent markers, to the sample. The antibodies linked up to the cells the researchers were interested in, and then the labelled cells were separated from the others. But the problem here stems from the method itself: The researchers succeeded in finding only the cells they were looking for from the start. Just like the fable about the five blind people and the elephant, in which each of them touches a different part of the animal and comes to a different conclusion.
Amit was convinced that in order to see the whole elephant, it was first necessary to go down to the level of the individual cell.
“The cells are the atoms of the body. Without getting to the level of the atoms, understanding will be very partial – we will not understand causality,” he says. “Until we know biology on the single cell level, we won’t know what’s going on.”
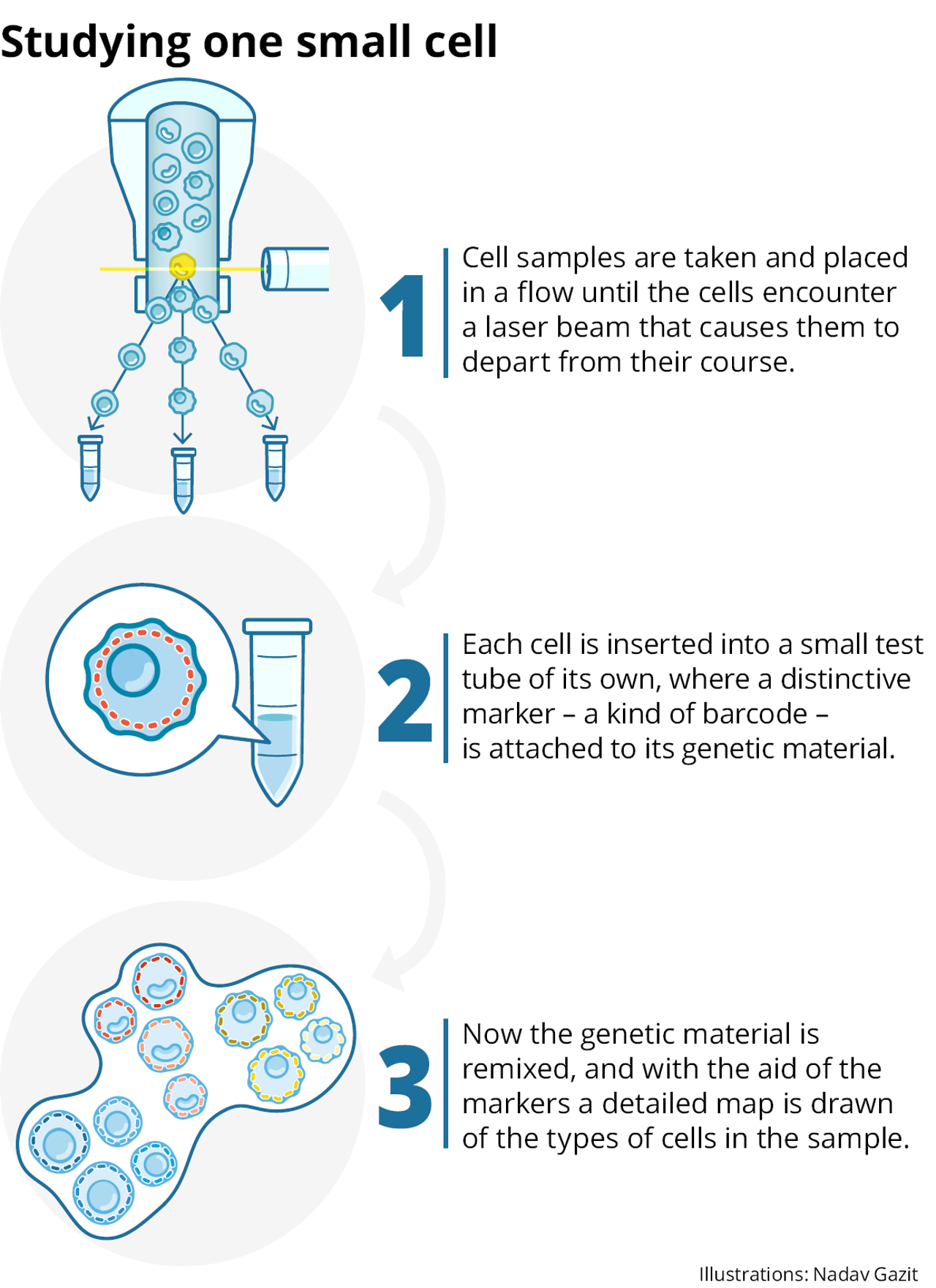
To that end, he decided to take the technology dating back to the 1980s, and apply it differently: not in order to label and focus on particular cells, but to separate all the cells in the sample and isolate and define each one separately.
“It was semi-suicide,” he says, “because at the time there wasn’t anything that was expected to work. The capabilities were remote, there was nothing. There was only the idea.”
He started to develop the idea during his last year in Boston. A year later he opened his lab in the Weizmann Institute, and in collaboration with Prof. Amos Tanay, from the institute’s mathematics department, he began to realize the vision.
Still no bad blood
A poster designed for the world’s first academic conference on single-cell genomics in 2012 showed a sketch of a car with all its parts laid out around it. The caption: “Sometimes the sum of the parts is greater than the whole.”
The field was in its infancy and the conference was perceived as something of an underground affair. But the event, which was held at the Weizmann Institute, created a community, or “sect,” as Amit puts it. Unusually in academia, this was a community that displayed a great deal of openness and sincere mutual compliments. “Probably that’s characteristic of the outset, when the potential is endless and there’s no competition for one’s place,” the professor notes. “And you also don’t have the bad blood that accumulates in older fields.”
The confab was the outcome of a persistent effort by Amit and his fellow researchers to encourage other laboratories worldwide to adopt their approach. “Our idea was so far-fetched that we didn’t succeed in persuading many people,” he says. “It didn’t sound practical, and it also didn’t sound like a problem worth investing in.” The typical response was: We know our cells. It’s not clear how this can help. Still, there were two scientists from Sweden, Sten Linnarsson and Rickard Sandberg, who accepted the invitation to attend the conference.
By the end of 2012, Amit and his colleagues had overcome the main problems, and the technology worked. For the first time it was possible to start studying the totality of the RNA molecules that exist in a single cell, and in a large number of cells in parallel.
The first article to describe the method was groundbreaking – and therefore took a long time to be accepted for publication. The journal Science finally published it in 2014, more than a year after its submission. “They tortured me with it, because it was the first time an idea like that had been proposed,” Amit recalls. “But it’s the basis for the direction everyone is moving in today.”
Once the principle was proved, Amit’s lab began to examine biological systems, ranging from the development of blood and immune system cells in the bone marrow and the activity of cells in the stomach, to viral processes such as flu infection and degenerative diseases. All told, Amit and his colleagues published more than 30 articles that shattered axioms and shed new light on well-known systems. “We found new types of cells, new states of cells and new pathways they exploit,” he says. “The technology generated a genuine explosion of new discoveries.”
The next turning point was a breakthrough that led to an understanding of the role played by the immune system in the development of Alzheimer’s disease. With the collaboration of Prof. Michal Schwartz from the Weizmann Institute, the scientists were able to identify a subtype of cells in the brain’s immune system, microglia, that are linked to development of the condition. A disruption in a receptor located on these cells makes them lose something of their effectiveness, leaving them unable to cope with the accumulation of waste in the brain.
They serve as the brain’s first line of defense against damage and are responsible both for communication with neurons and for disposal of waste and dead cells. Genetic studies found mutations that are characteristic of those suffering from the disease, but until Amit’s research the pieces did not fit together; it was not understood in which precise cells the gene had gone awry. “You have no idea how many mice were sliced and scraped, without identifying the relevant cells,” Amit says.
With the aid of a thorough examination of the brain cells of mice with an Alzheimer’s-like disease, the researchers succeeded in identifying the target cells. In patients with cell impairment, all waste that is created by random causes, such as stress or a sleepless night, can accumulate and bring about the death of additional neurons. And without the cleansing of the dead cells’ waste, a chain of responses is set in motion that can lead to Alzheimer’s. Once the cells are identified, studying them becomes much easier. “With a fulcrum you can move the world,” Amit says.
The discovery makes it possible to move ahead with methods to reawaken the cells that are intended to evacuate waste. “If we take people of 50 to 60 who are just starting to show the accumulation, and we help them with the cleansing, it will significantly halt the progress of the disease,” Amit says.
The problem is that the treatment can be relevant for the broader public only if the disease is detected at an early stage. Many efforts are currently underway to locate neural deterioration early on, but the research in these channels is still ongoing. Amit believes that developments based on his discoveries will make it possible to find a treatment for Alzheimer’s within the coming decade. As per the engineering metaphors he’s fond of, he compares the development of such treatment to the building of a car. “We have the engine, but we still need the wheels.”
This study was a turning point for him: “Until then, my goal was to develop the technologies and understand how things work. That was the first moment I said: We have a dramatic ability here that changes the potential to develop medications.” Since then, his lab’s research has focused on attempts to find solutions for different diseases, all the way up to the jewel in the crown of humanity’s problems: cancer.
Treasure of the lab
All the scientists who know Amit agree that he’s brilliant, but not everyone likes him. Even among those who work under him, there are those who say they were initially put off by him. “At first I rarely saw him smile,” says Dr. Shuang-Yin Wang, from China, a postdoctoral fellow who has been doing research in Amit’s laboratory for more than two years. “Later, when I really came into close contact with him, I learned that he’s a very nice person.”
Other scientists mention his distant, tough personality, and almost complete lack of humor. Many say he can be aggressive. Some have been burned by their experiences with him, and there are even those who feel that the power he has accumulated has blocked the path of others in the field.
And he has definitely accumulated power. Amit is the oracle of his field in Israel; he is involved in the vast majority of clinical studies here that utilize single-cell technologies. His laboratory, boasting some 30 researchers, is among the world’s leaders in its field in terms of publications, patents and the grants it receives. Its annual budgets total millions of dollars and it attracts brilliant scientists from around the world – from China and Poland to the Faroe Islands.
“The scientific achievements are leading to significant funding of studies,” says Amit, who adds, with a smile, that “maybe the most important thing is that the success attracts very creative people from Israel and from around the world, amazing minds who are not confined within the limits of the existing knowledge. So they don’t stop asking questions and creating new and unexpected findings – they are the laboratory’s true treasure.”

“Unexpected” is the name of the game here. Bjort Kragesteen, a postdoctoral fellow from the Faroe Islands, acknowledges that she came all the way to the Weizmann Institute because of Amit’s openness to wild ideas. She was actually set to do a postdoc closer to home – in Copenhagen – but was then told that problems had arisen in connection with her funding, because the idea she put forward was excessively risky. By chance she was touring Israel at the time, and her professor suggested that she email Amit. Within two days she met with him.
“I had only one slide in my presentation, an idea but no details, only the direction,” Dr. Kragesteen recalls. “He [Amit] told me, ‘That’s the best idea I’ve heard in a long time. You can start tomorrow. Money is no problem and you can do whatever you want here.’”
Indeed, one of the singular aspects of this lab in Rehovot, even by comparison to others abroad, is the diversity of the people and the fields of research it encompasses.
“Ido works on everything that his people want to work on,” says Aleksandra Deczkowska, from Poland, who is also doing a postdoc in Amit’s laboratory. “So what he has built is a playground for us – people passionate about very different things – to romp in.”
Dr. Deczkowska emphasizes that the professor has a special talent for sparking motivation among researchers – a particularly important adventure for a lab that has highly ambitious goals.
“There is a high chance of failure,” she says. “Because we do risky things, we need an environment that feels safe. I feel safe with Ido, because I feel that with him there’s no real failure. Ever. Because he will tell me, ‘Well, you learned something.’ It’s a safe playground.”
The tools, the funding and the talent of the researchers in his laboratory make it possible for Amit to set highly ambitious goals time and again. The most ambitious of these is to reshape the way medications and treatments are developed.
“The way we develop medications today is completely wrongheaded,” he says, adding that, with the exception of a very small number of medications, it is not actually known how the great majority of treatments work – that is, how exactly they influence cells and bring about a cure.
“The question is not what we think they are doing, but what they are doing in practice,” he says. “That is why we almost always don’t succeed in developing effective drugs, and if we do succeed, it’s often by chance, because we don’t understand the cellular intricacies and dynamic processes of cancer. When a medication works, it’s like a miracle is happening. But how does that miracle occur?”
Amit explains how single-cell technologies can help cope with the pitfalls affecting development of medications. When a company develops a medication today, it needs to choose from a large number of different molecules, one of which might produce the desired effect. Even afterward, the company must make dozens of decisions along the way to optimizing the molecule’s beneficial effects.
“And they are almost blind decisions,” Amit explains. “It’s a gamble.” The research methods he has pioneered make it possible to examine what actually happens to all the types of cell that make up the tumor when it is exposed to a certain drug – that is, how the medication affects a specific cell functioning in the tumor and its communication with other cells.
“Drug development is not doing very well,” agrees Prof. Miriam Merad, an expert in cancer immunology from Mount Sinai Hospital in New York who is collaborating with Amit in a study of lung-cancer patients. “The rate of stage-3 failures is sky-high,” she says, referring to the final state in testing during the process of drug approval.
Merad explains that these failures stem above all from the fact that treatments being tested are not sufficiently based on an understanding of the biology involved. She agrees that the tools being developed by Amit are having a dramatic effect in this regard. “Single-cell genomics is a transformative technology,” she says. “It allows biological information to be extracted from a small number of samples.” It is thus possible to make knowledgeable decisions about whether to continue to stage 3, and if so, to foresee who will benefit from the treatment and who will not. “It’s exciting, because we can reduce the time and the failure,” she says. “We hope to be faster, because we will be smarter.”
Rolls-Royce of treatments
In fact, many of the major international pharmaceutical firms have grasped the potential of Amit’s work, and have started to work with him. One of them is the German-based Merck Group. According to Dr. Joern-Peter Halle, the director of Merck’s preclinical research and development division, Amit “has the resolution to understand diseases better, and when you understand a disease, the better you can develop treatment for it.”
The Holy Grail of the cure for cancer lies in improving immunotherapy and in being able to predict who will respond to a particular treatment – to find the appropriate combination of treatments for each tumor in each patient. But immunotherapy is a process involving multiple changes, and the place and the time of every such change is of enormous importance. To understand how that process works, it’s not enough to explore each cell separately. Accordingly, the first challenge that Amit and his colleagues faced was to understand the connection between different cells.
To investigate the communication between cells, they need to be caught in the act. Amit and his lab partners have been able to devise a method that deciphers the spatial relations between different cells in the cancerous tissue and reveals how the interaction in the cells, in relation to the others, influences their activity.
But to understand what actually happens in the response to immunotherapy (as opposed to what we think happens), it’s also necessary to find a way to investigate what happens inside each individual cell. For that purpose, it’s essential to identify which proteins are active in each cell. To do that, antibodies can be injected into the cell, so they will identify the proteins and their activity. However, the moment the cell is punctured so as to allow injection of antibodies, the proteins activate the cell’s mechanisms of self-destruction.
Amit’s laboratory has succeeded in circumventing this predicament. The scientists have added materials that deactivate the proteins, thus taking them out of play. In this way the proteins can be marked and measured, and the genetic material can be analyzed intact. Using this method, it is possible to understand which activity is characteristic of which cell: what happens in each individual cell.
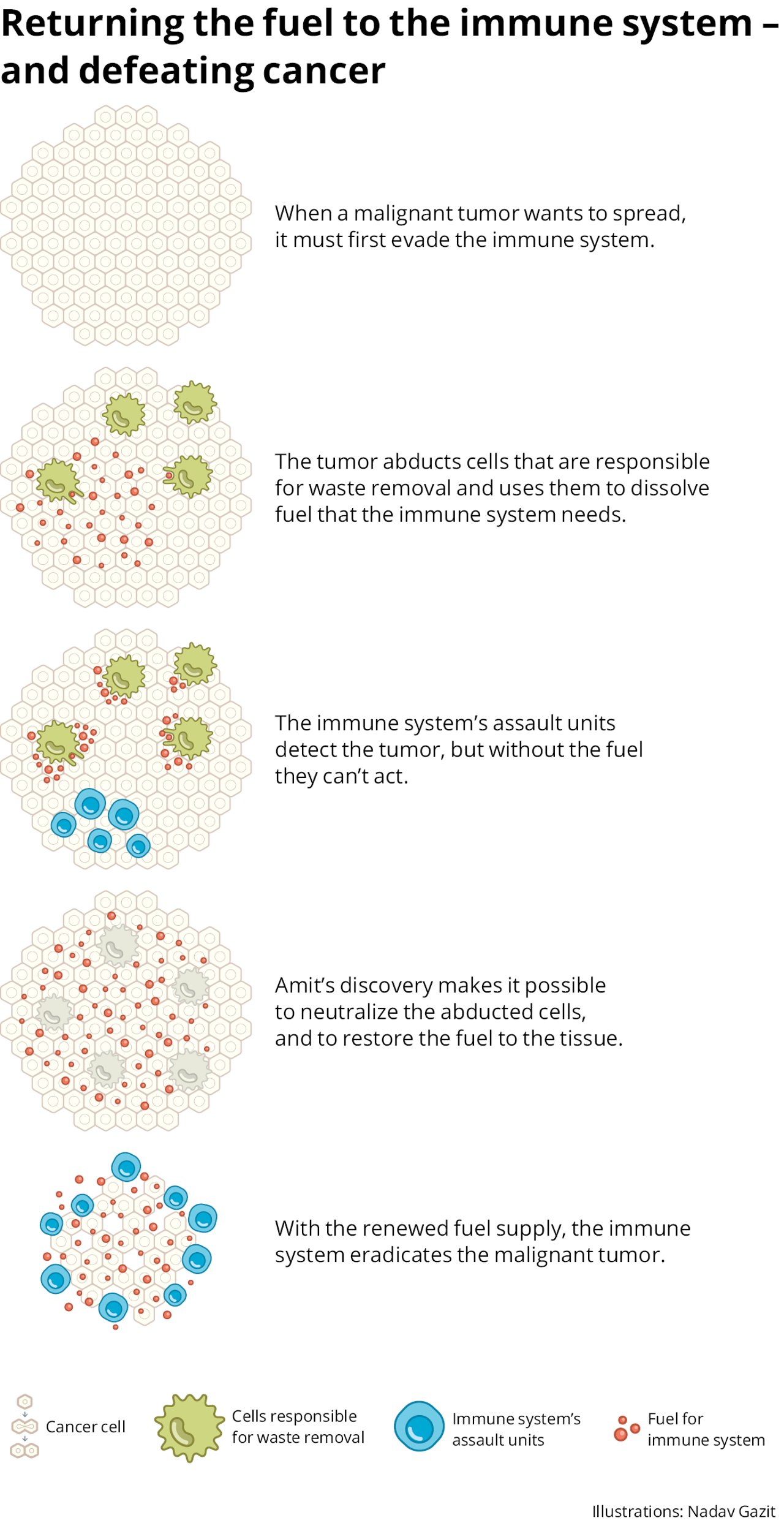
After developing these methods, Amit and his associates set out to discover which cells continue to interfere with the response of the immune system in its battle against cancerous growth, even after the use of recognized immunotherapy treatments. They knew that in order to combat the growth, the immune-system cells need “fuel” – a particular amino acid that exists in the medium between the cells. They knew also that other cells dismantle the fuel and thus do not allow the immune system to operate.
However, no one found the elusive cells that dismantle the fuel. “It was assumed that they exist, because their activity was seen,” Amit says. “But no one knew how to isolate them, how to explore them and how to disrupt their activity. I called them ghosts. One of the hottest things in the drug industry is to find out how to get rid of those cells, and thus to help reboot the immune system.”
The tools he and his lab developed made it possible to identify these cells for the first time.
Boarding the Beagle
To find the cells, Amit followed his usual procedure: He boarded the technological Beagle and sailed into the unknown. However, this time the research led him to the same receptor he had reached in the Alzheimer’s research. He also explains why: This immune receptor’s job is to sense its surroundings. If it detects tissue damage, it launches a chain reaction that curbs the operation of the immune system around it to prevent further damage. “The cancer hijacks this mechanism,” Amit explains. “This exists in almost all the types of cancer we studied.”
The joint research of Merck and Ido Amit began with biopsies of melanoma patients, both from those who responded to immunotherapy treatment, and those who didn’t. “We wanted to understand how the tumor protects itself and tricks the immune system,” he explains. They found that in melanoma, which responds well to immunotherapy treatments, a low proportion of the cells eliminate the fuel of the immune system. In other malignant tumors, those less responsive to immunotherapy, these cells are more widespread.
Amit: “We are now developing antibodies that are intended to block these cells. We are already in very advanced stages, and I hope this will lead to the development of medications quickly, hopefully within two-three years.”
But the insights gleaned from the cancer research don’t stop there. “We started the research with melanoma, but the principles we discovered are applicable to other diseases, too,” Halle says. “These mechanisms are more universal.” Thus, Merck is already using knowledge gleaned from Amit’s research to improve clinical experiments of treatments for other types of cancer.
“Something dramatic will emerge from it in the coming decade,” Amit says. “The things we are doing with the pharmaceutical companies will have an effect on patients within a few years.”
One of the fascinating examples of this derives from another type of cancer: multiple myeloma. In myeloma, a very common bone marrow cancer, one of the cells of the immune system multiplies uncontrollably in the marrow, which is essentially the factory of the blood. The disease causes damage to bones, to the manufacture of blood cells, to kidneys and more, and is fatal. The disease is currently incurable but treatable.
“Treatment of the disease has improved greatly in the past 20-30 years,” says Dr. Yael Cohen, director of myeloma services at Ichilov Hospital in Tel Aviv. “Today, the standard treatment extends the life of 80 percent of these patients by a decade or more. Throughout the treatment, one line of medication is administered, which is beneficial for some years. Then the cancer returns and a second line is given, and then a third, and so on. Some of the efforts are devoted to an attempt to develop more effective medications, in part with the aid of various immunotherapy treatments.
Amit worked with Cohen on answering a key question: why some of the patients don’t respond to treatment. To that end, they first checked patients on whom the first type of medications had no effect. Instead of giving them the second line of drugs, they were given a cocktail of the most effective medications from all the other lines.
“We wanted to give them the Rolls-Royce of existing treatments,” Dr. Cohen says. Here the subjects were divided into two groups: Those who responded to the treatment and those who suffered from “super-resistance” – even the highest quality cocktail didn’t help them. Using single-cell technologies, the scientists compared the cells of the patients who were resistant to the regular treatment and those who displayed super-resistance. By this means they identified a genetic pattern unique to the patients who were at especially high risk of not responding. They then examined the findings of an American database with information about nearly 1,000 myeloma patients. “What we saw in our 40 resistant patients made it possible to predict the prognosis of the 1,000 patients,” Cohen says.
The next, and most significant, stage was to understand which protein allows the cancerous cell to achieve resistance to medications. Finding the protein allowed the researchers to search for a molecule that would inhibit its activity. “We found a drug like that in the public database,” Cohen says. “It’s a drug that’s used for a different disease.” Cohen hopes that the clinical experiments will start in the coming months.
“The power of the research lies in the transition from the medical need to the laboratory, and from there back to the patient’s sickbed,” she says.
The study originated in the clinical need of physicians to understand the reason for the resistance to treatment, moved to the laboratory to decipher the mechanisms behind the medical problem, and then returned to the clinic to test the proposed medication. Both Dr. Cohen and Dr. Halle are convinced that this track is a dramatic engine for scientific progress.
Many-tentacled monster
Earlier this century, following completion of the Human Genome Project, it was widely believed that possessing the “manual of life” would foment a medical revolution and consign to oblivion the major diseases threatening humanity. Within about a decade it had become clear that behind the complexity of the genetic code lie multiple other complexities, and that deciphering the code is only one step in a long journey to understanding human biology – and why the biology goes wrong.
What applies to human biology overall holds for cancer specifically. “Cancer is an ocean, it is actually more than 200 diseases, so there will never be one cure for cancer,” says Prof. Neta Erez, head of the pathology department of Tel Aviv University’s Sackler School of Medicine, and an expert in the research of the micro-environment of cancer. “And even within an individual tumor there is great complexity. It’s a many-tentacled monster.” This is one reason why many people are likely to be skeptical of the notion that one technology, just a decade old, will be able to triumph over cancer.
Indeed, for the single-cell technologies to be able to transform thoroughly the treatment of cancer, they will have to overcome a host of practical obstacles. “Each of the obstacles can be addressed, but together they leave the road ahead unclear,” says Lior Pachter, an expert in computational biology at the California Institute of Technology. Beyond this, Prof. Pachter adds, “cancer is what happens when something goes awry in biology. In order to ‘solve’ cancer, it’s necessary to understand the whole biology of human development from a single cell. It’s not certain that one technology will make that possible.”
At present, Prof. Aviv Regev and Dr. Nir Hacohen, both of whom Amit worked in his postdoc, are among the scientists spearheading the development and application of single-cell technologies in the United States. Other researchers, including the Swedish scientists Linnarsson and Sandberg, are working on the subject in various locales internationally – each laboratory with its research platform. However, Moshe Sade-Feldman, director of the Translational Cancer Immunology laboratory at Massachusetts General Hospital, Boston, and others point to significant difficulties that will have to be overcome for Amit’s methods to be able to revolutionize the approach of physicians to cancer patients.
Consider the cost and complexity of the method. Dr. Sade-Feldman explains, for example, that the cost of analyzing a single sample can run to thousands of dollars and that the technology is so sensitive that any small technical mistake in handling the sample renders the results unusable. In addition, there are cells that respond to their removal from the body. Incautious handling or a delay could cause important information to be lost, or lead to the wrong conclusions being drawn. That’s one of the reasons that Sade-Feldman’s laboratory is located in a hospital. The proximity allows for close work with the oncological teams and a rapid processing of the samples the moment they are removed from the body. This infrastructure can be highly advantageous, but requires an immense budget.
The technology also faces another formidable challenge: the fact that it cannot “see” things in real time.
“The moment you arrive at a single cell, you kill the tissue, it’s already not living,” notes Prof. Nadav Ahituv, who heads a laboratory that investigates gene regulatory elements and their relationship to human diversity and disease at the University of California, San Francisco. “You are actually looking at one point in time; it’s like a picture, it’s not a video.”

If Amit and his associates present methods for studying single cells across the dimension of time as well, as the Weizmann Institute scientists thinks he is capable of doing, Ahituv agrees that this will make it possible to offer “personalized medicine” for cancer patients: a fusion of treatments best suited to the particular patient’s tumors. These focused treatments can transform immunotherapy from an approach that is beneficial to only some patients and a small number of types of cancer, into a broad and far more effective method of treatment.
There are some who are even more optimistic. “It is the forefront of the technology,” says Merck’s Halle. “I am pretty certain that in another 10 years the single-cell methods will not be used only in academic and research contexts but in clinical laboratories as well. Better understanding will accelerate the development of medications, but the technology will also be routine, and that is what is most important. There will not be one person who will cure cancer, but Ido is leading in the field and giving inspiration to others.”
Miriam Merad, from New York, agrees. “To say that this technology will revolutionize medicine is absolutely a valid claim,” she asserts. “Ido is making us go faster, because he constantly pushes the boundaries. He is extremely ambitious – not at the personal level: He is ambitious with his science.”
Amit is aware of the scale of the challenges, but is not deterred. “With every new technological invention, we are moving forward, with giant leaps, in understanding cancer and how its mechanisms evade the immune system. Cancer, in contrast, is not inventing new mechanisms; it’s the same cancer for thousands of years,” he sums up. “Cancer is still ahead in the race. But occasionally we succeed in catching up with it, and that is happening more and more. In the end we will defeat it, that’s clear.”
