
REHOVOT, ISRAEL—April 14, 2021—Being constantly hungry, no matter how much you eat – that’s the daily struggle of people with genetic defects in the brain’s appetite controls, and it often ends in severe obesity. In a study published in Science, researchers at the Weizmann Institute of Science, together with colleagues from the Queen Mary University of London and the Hebrew University of Jerusalem, have revealed the mechanism of action of the master switch for hunger in the brain: a receptor called melanocortin 4 (MC4). They have also clarified how this switch is activated by setmelanotide (brand name Imcivree), a drug recently approved for the treatment of severe obesity caused by certain genetic changes. The team’s findings shed new light on the way hunger is regulated and may help develop improved anti-obesity medications.
The MC4 receptor is present in a brain region called the hypothalamus, where it is in a cluster of neurons that compute the body’s energy balance by processing a variety of energy-related metabolic signals. When MC4 is activated, or “on” – as it normally is – it sends out commands that cause us to feel full, which means that, from the brain’s perspective, our default state is satiety. When our energy levels drop, the hypothalamic cluster produces a “time to eat” hormone that inactivates, or turns off, the MC4 receptor and sends out a “become hungry” signal. After we eat, a second “I’m full” hormone is released. It binds to the same active site on MC4, replacing the hunger hormone, turning the receptor back on, and bringing us back to the satiety default.
Mutations that inactivate MC4 cause people to feel constantly hungry. The receptor is a prime target for anti-obesity drugs, such as setmelanotide, precisely because it’s a master switch: turning it on can control hunger while bypassing all other energy-related signals. But until now it was unknown how exactly this hunger switch works.
The new study began with the predicament of one family in which at least eight members, plagued by persistent hunger, were severely obese. Most of them had a body mass index (BMI) of over 70 – about triple the norm. Their medical history came to the attention of Hadar Israeli, a medical student pursuing PhD studies into the mechanisms of obesity under the guidance of Dr. Danny Ben-Zvi at the Hebrew University of Jerusalem. Israeli was struck by the fact that the family’s plight was due to a single mutation: one affecting the MC4 receptor. She turned to Dr. Moran Shalev-Benami of Weizmann’s Department of Chemical and Structural Biology, asking whether new advances in electron microscopy could help explain how this particular mutation could produce such a devastating effect.
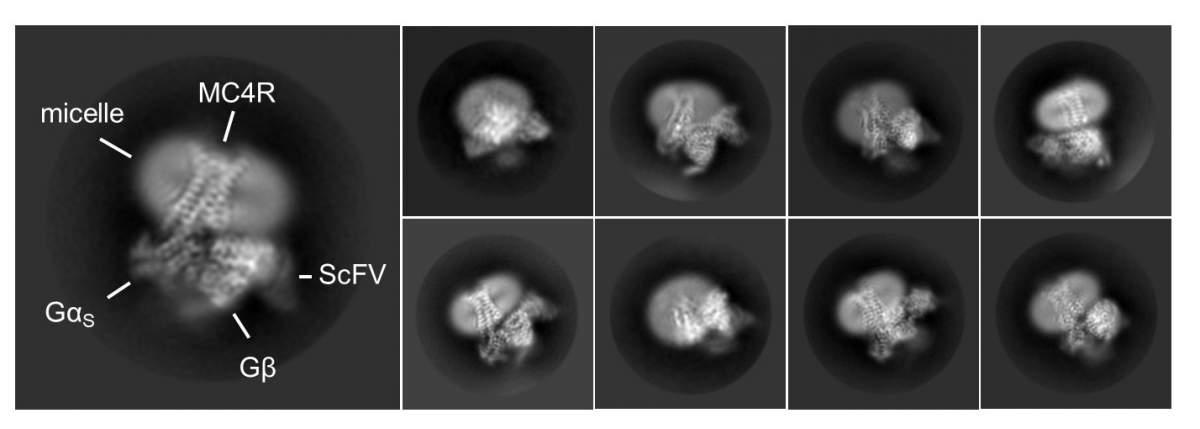
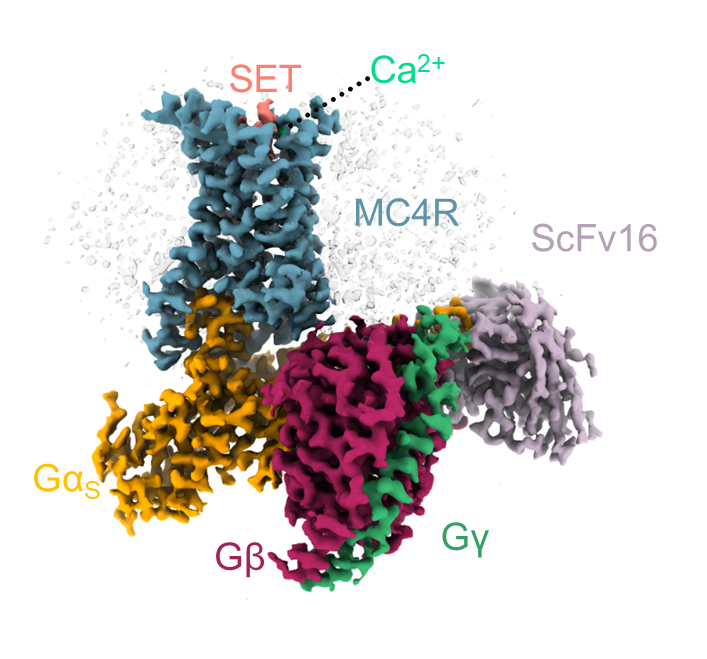
Dr. Shalev-Benami decided to launch a study into the structure of MC4, inviting Israeli to join her lab as a visiting scientist. Together with Dr. Oksana Degtjarik, a postdoctoral fellow also in the lab, Israeli isolated large quantities of pure MC4 receptor from cell membranes, let it bind with setmelanotide, and then determined its 3D structure using cryogenic electron microscopy. The study was conducted in collaboration with the teams of Dr. Peter J. McCormick from the Queen Mary University of London and Prof. Masha Y. Niv from the Hebrew University of Jerusalem.
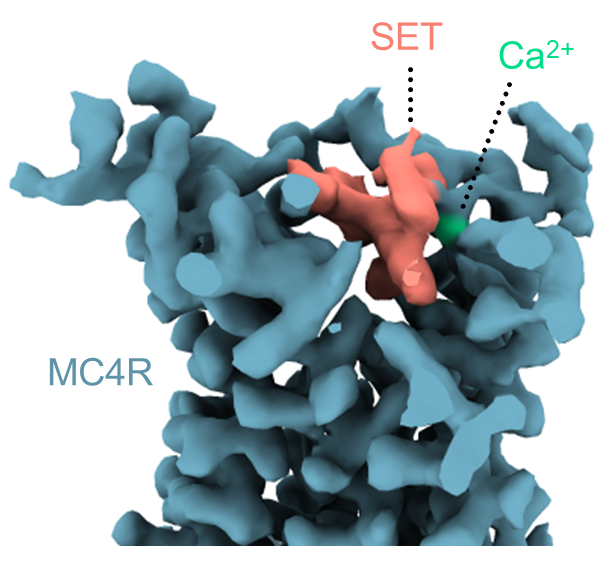
The 3D structure revealed that setmelanotide activates the MC4 receptor by entering its binding pocket – that is, by directly hitting the molecular switch that signals satiety, even more potently than the natural satiety hormone. It also turned out that the drug has a surprising helper: a calcium ion that also enters the pocket, enhancing the drug’s binding to the receptor. In biochemical and computational experiments, the scientists found that, similar to the drug, calcium also assists the natural satiety hormone.
Prof. McCormick states that “Calcium helped the satiety hormone activate the MC4 receptor while interfering with the hunger hormone and reducing its activity.”
“This was a truly unexpected finding,” says Dr. Shalev-Benami. “Apparently, the satiety signal can successfully compete with the hunger signal because it benefits from the assistance of calcium, which helps the brain restore the ‘I’m full’ sensation after we eat.”
MC4’s structure also revealed that the entry of setmelanotide causes structural changes in the receptor; these changes appear to initiate the signals within the neurons that lead to the sensation of fullness. The study has explained how mutations in the MC4 receptor can interfere with this signaling, leading to never-ending hunger and, ultimately, obesity.
Moreover, the scientists have identified hotspots that crucially distinguish MC4 from similar receptors in the same family. This should make it possible to design drugs that will bind only to MC4, avoiding side effects that may be caused by interactions with other receptors.
“Our findings can help develop improved and safer anti-obesity drugs that will target the MC4 receptor with greater precision,” Dr. Shalev-Benami says.
Study participants included Dr. Fabrizio Fierro of the Hebrew University of Jerusalem; Vidicha Chunilal, Amandeep Kaur Gill, Nicolas J. Roth, Dr. Joaquin Botta, and Dr. Li F. Chan of the Queen Mary University of London; Dr. Vadivel Prabahar from the Weizmann Institute’s Chemical and Structural Biology Department; and Dr. Yoav Peleg of Weizmann’s Life Sciences Core Facilities Department.
Dr. Moran Shalev-Benami’s research is supported by the Tauro Career Development Chair in Biomedical Research; the Ilse Katz Institute for Material Sciences and Magnetic Resonance Research; the Zuckerman STEM Leadership Program; the Joseph and Wolf Lebovic Lab; and the Abisch Frenkel Foundation for the Promotion of Life Sciences.
